by Dr. Sanjay Gadasalli, UT Southwestern Medical Center, Medical City Dallas
Sanjay Gadasalli is an aspiring pathologist and technology enthusiast who stays up to date on innovative technologies with the potential to impact clinical practice. While he has not personally worked with 3D histology, he has read studies whose methodology included it and has engaged in discussions with companies that develop it. He shares his review and outlook of the technology here.
Pathology is undergoing multiple technological revolutions. First, there is the wave of slide digitization that is enabling new opportunities in clinical diagnosis and education. AI and deep learning are another major development. But there are other, lesser-known technologies being developed that have the potential to change how pathology is practiced in the future. Among these is 3D histology technology.
What is 3D histology?
Simply put, 3D histology is any process that allows histological visualization of tissues in their original structure - as opposed to a mere 2D section of a tissue. I say “any process” because there are multiple. Traditional compound microscopy is not used, but rather several newer microscopy modalities, such as confocal, multiphoton, and light-sheet (“LSM,” the focus of this article).
In all cases, after a specimen of appropriate size is resected (because it is still not feasible to process large specimens), the tissue is fixed in formalin just like it would be for 2D histology. From there the process starts to differ.
Because the tissue needs to be imaged thoroughly into its deeper layers (in the case of LSM, by sheets of light), it must first be “cleared” to make it transparent. This involves using clearing agents like CLARITY, CUBIC, or iDISCO to remove lipids and other components that cause light scattering. Then the specimen is stained, optically imaged in sequential thin sections, and reconstructed into a 3D model.
The figure below shows how light-sheet imaging is performed. Fluorescence emitted from the illuminated plane of tissue is detected perpendicularly to the plane. This orthogonal setup reduces background noise and improves signal-to-noise ratio.
From there, the acquired images need to be processed to enhance quality, remove noise, and correct any artifacts. After that, specialized software reconstructs the series of 2D images into a 3D model. The 3D model is then analyzed to extract relevant information, including structure quantification and spatial relationships.
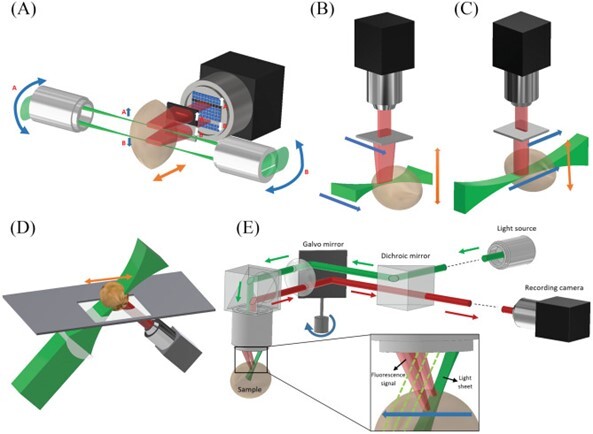
(Created by Delgado-Rodriguez et al., shared with permission by the license type. Find more info at the end of this article.)
What are the proposed benefits, advantages, and opportunities?
If you think about it, there is a drawback to 2D histology in that data is limited or lost. Three-dimensional tissue is reduced to an arguably simpler two-dimensional slide. 3D histology therefore provides a complete view of tissue architecture, allowing pathologists to understand the spatial relationships between cells and structures in their native context. And it reduces the risk of missing critical structures. Imagine not needing to recut deeper sections!
When it comes to areas like cancer diagnosis, better delineation of tumor boundaries and margins can lead to improved surgical planning and prognostic assessment. And enhanced detection of small metastases and infiltrative growth patterns could catch details that might be missed in 2D sections.
Finally, it synergizes more with AI applications such as cell detection & quantification.
The bottom line would be enhanced diagnosis. One can also make the argument that with advances in automation, time and financial savings would be added to the list.
Major challenges to implementation and next steps
All novel technologies face similar hurdles: regulatory approval, cost-benefit analysis, and passing validation tests. Given that 3D histology also represents a relatively novel diagnostic modality, it faces additional challenges:
- Optimizing the technology itself - for example, errors in compiling single slices into 3D reconstructions have plagued early attempts to reproduce reliable results.
- Cost and availability of technology – For example, light-sheet microscopy is currently only offered at 25 centers around the world (see below). These setups can be prohibitively expensive for many institutions. Unfortunately, the other modalities (confocal or multiphoton microscopes) can be equally expensive. A comparison between these microscopy methods is beyond the scope of this discussion but could be the subject of a future post.
- Enough advantages to overcome barriers.
- Effort to learn new technology and train pathologists in the interpretation of 3D histology. As an aspiring pathologist, I am personally excited by this prospect but it does represent a challenge in otherwise busy training programs.
- For companies developing the technology, regulatory approval is a familiar challenge, as many of these technologies are classified as medical devices.
With these in mind, the next steps for 3D histology technology are:
- Continued optimization and minimization of errors.
- Comparative studies and clinical validation to ensure that 3D histology provides clinically relevant information that improves diagnostic accuracy and patient outcomes, and adds value over traditional 2D methods.
- Creating robust (and eventually affordable) data storage, management, and processing infrastructure. As well as integrating 3D histology into existing clinical workflows.
- Creating and integrating 3D histology interpretation into training programs. – A few medical schools have begun trialing 3D histology tools for educating medical students, although the practice is far from widespread.
- Technology feedback from pathologists. – This step is crucial in the development of any new technology or clinical tool.
Guidance for adoption and implementation
Some pathologists may find themselves at large centers (e.g. academic) with the capacity to lay the foundation for eventual adoption. They can do several things to lay the groundwork.
First, they can start to generate interest. There is a growing body of evidence showing the efficacy of 3D histology, and webinars or lectures sharing this evidence can alert leadership and trainees to what the future holds. This applies not only within the pathology department, but also to external stakeholders. For example, involving colleagues in surgery and administration has been a crucial step for the adoption of whole slide imaging.
Next, these early adopters will need to acquire the necessary materials and technology - most feasibly, by partnering with centers that already have the technology that enables 3D histology. For instance, The mesoSPIM Initiative (mesospim.org) lists the 25 centers around the world with light-sheet microscopy setups. Alternatively, there are multiple companies that can walk an institution through the process.
Finally, academic institutions will need to start developing educational and training materials so that current and future pathologists (like myself) can increasingly engage with the new modality.
Looking forward
As with all new technology, the outlook is bright but filled with hurdles. 3D histology won’t be a game-changer like the original microscope was, and it certainly won’t be useful in all areas of pathology. But if used appropriately, it may likely prove its worth. Like other novel technologies, it will probably be used in research settings before it finds widespread clinical use. After that, large institutions will be the ones to trial it out, while deferring to the 2D histological results in cases of discrepancy. As an aspiring pathologist, I am excited at the prospect of using this technology to better guide my colleagues’ treatment decisions and provide more accurate diagnoses for my patients. However, those outcomes are yet to be proven and come with their own costs.
To recap, LSM-enabled 3D histology is a novel modality that uses a complex process (and expensive technology) to allow pathologists to analyze tissue in three dimensions. While it has many potential advantages and could open new doors, it faces a myriad of hurdles. However, with prudent adoption practices, it will likely find at least a few areas of application and improve the lives of some people.
References
- https://www.sciencedirect.com/science/article/abs/pii/S1361841518300264
- https://www.science.org/doi/10.1126/science.1068206?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed#
- https://www.sciencedirect.com/science/article/pii/S000294401200168X
- https://www.sciencedirect.com/science/article/abs/pii/S1361841518300264?via%3Dihub
- https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fasebj.2018.32.1_supplement.25.5
Image permissions:
The material is available under Public License (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Pablo Delgado-Rodriguez, Claire Jordan Brooks, Juan José Vaquero, Arrate Muñoz-Barrutia.
Innovations in ex vivo Light Sheet Fluorescence Microscopy.
Progress in Biophysics and Molecular Biology, Volume 168, 2022, Pages 37-51, ISSN 0079-6107.
https://doi.org/10.1016/j.pbiomolbio.2021.07.002.
https://www.sciencedirect.com/science/article/pii/S007961072100081X

Disclaimer: In seeking to foster discourse on a wide array of ideas, the Digital Pathology Association believes that it is important to share a range of prominent industry viewpoints. This article does not necessarily express the viewpoints of the DPA; however, we view this as a valuable point with which to facilitate discussion.
1 comment(s) on "3D Histology: Opportunities & Challenges to Implementation"
09/11/2024 at 06:07 PM
Sathya Srinivasan says:
The post deals with the imaging and image reconstruction approaches. 3D information can be obtained from 2D data using methods like Stereology. I am not seeing much interest from pathologists to apply stereology principles which will be an interesting way of looking at things. Stereology is more efficient, unbiased and informative when done correctly to get 3D information from 2D data.Please log in to your DPA profile to submit comments