by the DPA Reimbursement Task Force
Key Takeaways
- Thirty new Category III digital pathology codes were approved by the AMA and will become effective January 1, 2024, expanding the number of pathology services for which digitization can be reported separately to 43 services.
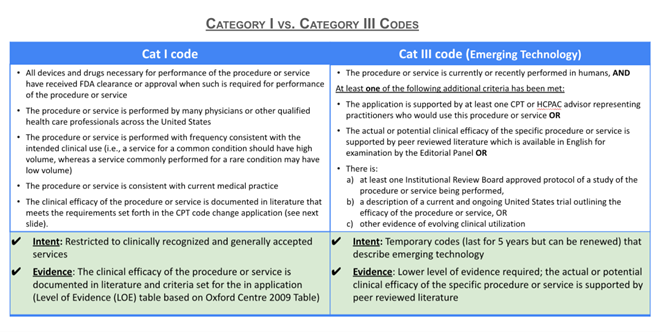
- Like the original 13 codes that became effective January 1, 2023, the new codes are intended to be used to report the additional work required for glass slide scanning and digitization. However, also like the original set of codes, no national payment rates (i.e., Relative Value Units) have been assigned to these codes.
- Widespread use in clinical practices will need to be demonstrated before the new codes can be considered for transitioning to Category I codes and assigned a national payment (i.e., RVUs).
- Initial feedback from the field indicates limited progress to date in implementing the slide digitization codes across pathology practices and health systems and highlights barriers inhibiting widespread adoption of the slide digitization codes, including lack of a national payment rate.
- The DPA Reimbursement Task Force encourages pathology labs and their institutions universally to ask the payers to whom they submit claims how such codes should be used and whether they are reimbursable. Labs are encouraged to implement standard operating procedures to consistently report the 43 Category III digital pathology codes (the 13 original (effective January 1, 2023) and the 30 new (effective January 1, 2024)) in line with payer guidance.
Thirty New Category III Slide Digitization Codes Expand On Original 13
As an update to a previous Blogpost by the DPA Reimbursement Task Force on August 8, 2022, the DPA Reimbursement Task Force is pleased to announce the approval by the AMA Editorial Panel on June 2 of 30 additional Category III slide digitization codes effective January 1, 2024. This announcement comes on the heels of the first year of implementation of the original thirteen Category III codes that were accepted at the May 2022 Editorial Panel meeting and effective January 1, 2023, totaling the number of Category III slide digitization codes to 43. The new cohort of Category III codes expands the number of pathology services for which digital pathology service can be reported (including cytopathology [varying by slide preparation methods, staining techniques or surgical applications], in situ hybridization, tissue blocks, blood smear, bone marrow, electron microscopy) and use cases for which it can be applied (including consultation [varied by where preparation occurred and the context in which it is being performed] and retrieval from archives for additional molecular testing), but are not structurally different from the original thirteen slide digitization codes.
Implications of the Category III Codes
Figure 1 presents the three types of codes for pathology physician services (Category 1, Category III, and HCPCS Level II) available for payment rate determination under CMS. Codes for laboratory testing (e.g. MAAA and PLA codes) are not included. The establishment of Category III codes for glass slide digitization can:
- Build awareness in both the AMA and CMS regarding the expansion of utilization and digital pathology
- Establish a coding foundation from which reimbursement for digital pathology can potentially be addressed
- Provide a mechanism for appropriately reporting not only the additional capital equipment costs, but also the increased time requirements and maintenance costs associated with whole slide imaging.
Category III codes are not associated with a payment rate, but the pathology community has been interested in establishing Category III slide digitization codes as a way to demonstrate utilization of digital pathology to support future creation of Category I codes which would then be assigned Relative Value Units (RVUs) resulting in a national payment rate. We readers refer to a forthcoming blog post entitled “Pathways to Direct Reimbursement of Digital Pathology/AI Technology ” for a more in depth explanation of some limitations posed by Category 1 coding for digital pathology and examples of alternative pathways in which CMS has (1) assigned payment rates for Category III codes, (2) established alternative HCPCS Level II codes with payment rates, and (3) elected not to follow AMA’s RUC recommendations for code valuation and instead selected alternative valuation methods.
-
[1] Under the Physician Fee Schedule (PFS).
[2] PFS payment rates are calculated based on the summation of the a professional component and a technical component subject to geographic and policy adjustments The professional component is based on a summation of the relative value units (RVUs) of physician work, practice expense (PE) and malpractice expense (MP) multiplied times a conversion factor. While RVUs are set by CMS, recommendations of the AMA’s Relative Value Scale Update Committee (RUC) are given consideration. The RUC does not recommend RVUs for Category III codes, and therefore CMS may have limited or no information available to assign payment rates to Category III codes unless such information is provided by other sources.
-
Barriers to Claims Reporting
Initial feedback from the field indicates limited and uneven levels of integration of the original thirteen Category III codes across pathology practices and health systems thus far and highlights stumbling blocks and barriers to adoption. Surveys of participants at this year’s Executive War College and a committee of the National Society for Histotechnology (HistoQIP) as well as individual feedback by DPA members reveal the following challenges faced in integrating reporting of the Category III slide digitization codes:
- System integration, system incompatibility and IT interface issues
- Lack of endorsement by institutional leadership and decision-makers
- Insufficient knowledge and understanding of the codes
- Underreporting and overreporting of codes
- Confusing to payers
- Fear of and/or experience with having claims rejected
Such impediments are inhibiting widespread uptake of these codes. In addition, the feedback from these sources shares a common theme - that a major obstacle disincentivizing adoption of the new codes is the lack of an associated payment rate.
How Is The DPA Reimbursement Task Force Making A Difference?
The DPA is playing an active role in seeking policy actions that appropriately reimburse digital pathology and AI services and has recently initiated engagement with CMS on these issues. The DPA reimbursement Task Force is meeting with CMS this quarter to introduce the DPA organization, educate CMS on the value of digital pathology/AI, and advocate for key policy changes to:
- Remediate payment policies in which digital pathology AI services are currently packaged into the payment for a primary service; and
- Establish appropriate and separate valuation of digital pathology services distinct from the primary pathology service (e.g., by assigning payment rates to existing Category II codes).
The DPA Reimbursement Task Force is collaborating with other stakeholder organizations who are in alignment with DPA to spearhead or jointly sign on to policy advocacy letters. The Task Force plans to submit comment letters to CMS and Medicare Administrative Contractors in response to regulatory, coverage, coding and payment policies that impact digital pathology/AI such as those put forth in the proposed and final rules for the Physician Fee Schedule and the Hospital Outpatient Prospective Payment System. The DPA also plans to initiate or support coding initiatives that maximize the opportunities for reimbursement that align with CMS evolving policies.
How Can the Digital Pathology Community Help?
First, the DPA Reimbursement Task Force strongly encourages the digital pathology community to fully integrate the Category III slide digitization codes (which now total 43 codes as of January 1, 2024) into practice protocols and claims systems. We also encourage the pathology community to communicate to DPA successes and challenges with integrating the Category III codes into practices.
Second, the DPA urges its members and the pathology community to monitor and understand the implications of AMA coding developments and CMS policy and rulings that affect coverage, coding and payment policy for digital pathology and AI software services. We recommend that the pathology community review the forthcoming blog post entitled "Pathways to Direct Reimbursement of Digital Pathology/AI Technology ".
Third, if CMS decides to or is persuaded to assign payment rates for slide digitization Category III or Category 1 codes, CMS will need supporting data to assign these payment rates. It is up to other stakeholders in the industry to (1) provide that data, similar to the information the AMA’s RUC provides (e.g., in the form of invoices for equipment and time allocation/time requirements) or (2) suggest crosswalk codes that approximate the additional capital expense and time associated with slide digitization. The stakeholder community can be poised to provide such information proactively to AMA’s RUC or in response to CMS requests for industry input and comment.
In a world of rising health care costs, it is critical to show that any innovation associated with new costs brings value. Stakeholders must communicate that value so that policy makers and payers are aware of the clinical utility of slide digitization.
Figure 1
Coding Systems
Applicable to Pathology Services, Laboratory Tests, and Digital Pathology/AI Technologies
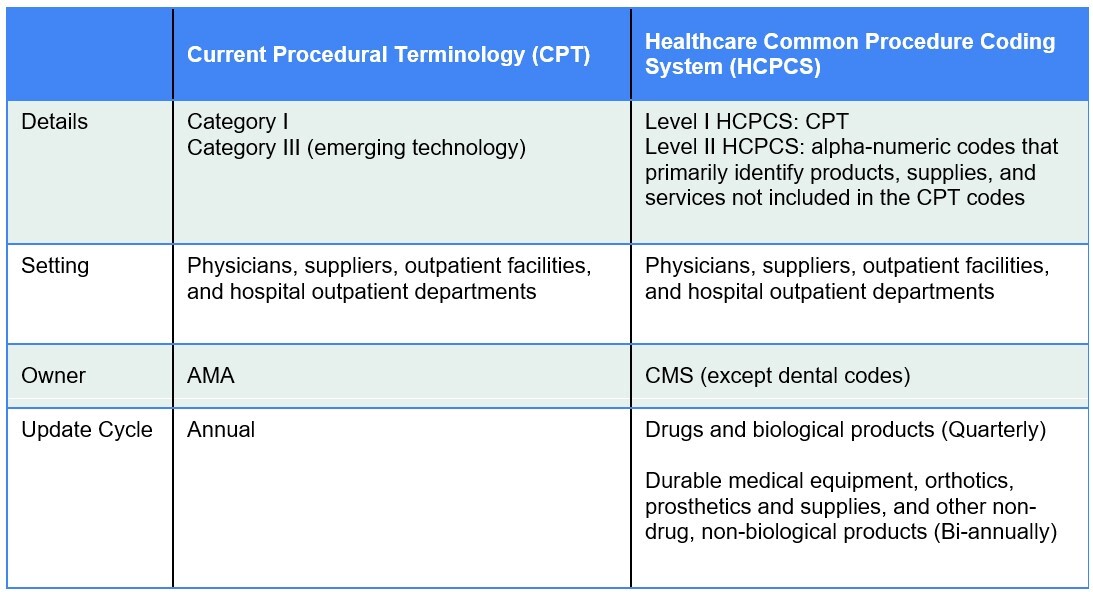
Figure 2
*****
The DPA Reimbursement Task Force pledges to contribute actively to the momentum towards obtaining reimbursement for digital pathology services and to communicate developments to the pathology community. The DPA strives to build an effective and unified advocacy effort for increasing adoption of digital pathology/AI and ensuring reimbursement of these services. Digital pathology’s reimbursement future has yet to be charted, and the DPA will continue to seek ways to influence coding, coverage and payment decision-making by policy makers on behalf of its members.
Please contact the DPA Reimbursement Task force info@digitalpathologyassociation.org
Disclaimer: In seeking to foster discourse on a wide array of ideas, the Digital Pathology Association believes that it is important to share a range of prominent industry viewpoints. This article does not necessarily express the viewpoints of the DPA; however, we view this as a valuable point with which to facilitate discussion.
Please log in to your DPA profile to submit comments