by the DPA Reimbursement Task Force
Key Takeaways
- Thirteen slide digitization Category III codes are in effect currently, and 30 more will become effective January 1, 2024. (See DPA Blog Additional Category III Slide Digitization Codes Signal Continued Evolution of Coding and Valuation in Digital Pathology). These codes are intended to be used to report the additional work required for glass slide scanning and digitization. No payment rate is associated with the codes.
- It remains unclear how CMS and many other payers perceive these codes, which may be a major factor hindering code use on claims up to this point.
- A primary rationale behind stakeholder endorsement of these codes is to demonstrate the utilization of slide digitization sufficiently to support the transition to Category 1 coding.
- Conversion of a Category III code to a Category I code for pathology services has both potential advantages and disadvantages of which the digital pathology community should be aware.
- While obtaining a payment rate via a Category I code is the more traditional process for obtaining payment, CMS has independently established national payment rates for Category III, Category 1 codes (not following AMA’s Relative Value Scale Update Committee (RUC) recommendations), and has also created alternative HCPCS Level II codes to be used in lieu of CPT codes for services.
- DPA advocacy and input by the pathology community can help drive the effort to obtain payment rates for slide digitization through existing coding or alternative coding structures and also by providing resource valuation data to CMS and AMA’S Relative Value Scale Update Committee.
Reimbursement Implications and Limitations of Category III Codes and Category I Codes
Thirty new Category III digital pathology codes were approved by the AMA and will become effective January 1, 2024, expanding the number of pathology services for which digitization can be reported separately to 43 services. Like the original 13 codes that became effective January 1, 2023, the new set codes are intended to be used to report the additional work required for glass slide scanning and digitization. However, also like the original set of codes, no national payment rates (i.e. Relative Value Units (RVUs)) have been assigned to these codes.
The pathology community has been interested in establishing Category III slide digitization codes as a way to demonstrate utilization of digital pathology to support future creation of Category I codes which would be assigned Relative Value Units (RVUs) under the Physician Fee Schedule (PFS) resulting in a national payment rate. (See Figure 1 for a description of Category III, Category I and Level II HCPCS codes) PFS payment rates for pathology services are calculated based on the summation of a professional component and a technical component subject to geographic and policy adjustments The professional component is based on a summation of the relative value units (RVUs) of physician work, practice expense (PE), and malpractice expense (MP) multiplied times a conversion factor (Figure 2 and Figure 3). While RVUs are set by CMS, recommendations of the AMA’s RUC are given consideration. The RUC does not recommend RVUs for Category III codes, and therefore CMS may have limited or no information available to assign payment rates to Category III codes unless such information is provided by other sources.
To date, the stakeholder community has rallied around the goal of converting the Category III slide digitization codes to Category I codes as a seemingly logical first step to obtaining reimbursement for digitization, but it is not clear yet that Category I codes are either necessary or sufficient for reimbursement. Coding for digitization can be a key ingredient in the process to obtain recognition for digital pathology and to demonstrate its utilization, setting the stage for reimbursement, but it is important for the DPA community to consider the following:
- Category III codes are frequently reimbursed, even if this is via local payment rate assignment
- Category I codes may not necessarily be given a more favorable payment rate than their Category III counterparts.
CMS alone, not AMA, has the authority to assign payment for digital pathology pathologist services and laboratory testing (under PFS) and for digital pathology/AI technologies (under the Hospital Outpatient Prospective Payment System)(Figure 4). CMS and AMA are not always aligned on coding or resource requirements underlying payment rates. Below we explain some challenges and risks associated with conversion of Category III slide digitization codes to Category 1 codes. We also provide examples in which CMS has exercised its authority to (1) establish payment rates for Category III codes, (2) establish alternative HCPCS Level II codes not aligned with codes established by the AMA, and (3) elect not to follow RUC recommendations for code valuation (i.e., the technical component, practice expense or both) and instead selected alternative valuation methods.
Challenges And Risks Associated With Category 1 Codes
The threshold of evidence required for Category I coding is substantially greater than for Category III codes and can sometimes take years to obtain. Figure 5 contrasts the requirements for Category III and Category I codes. Numerous Category III codes have remained Category III codes for a decade or longer, raising questions as to whether a Category I code will ever be created for the service.
The timeframe for conversion may be lengthy, and provide only limited visibility into the likelihood of success throughout the process. CMS allows for up to five years before a Category III code gets archived if it has not been converted into a Category I code. This means that tracking and evidence generation may continue throughout this period without a definitive sense as to the ultimate likelihood of conversion.
If a code change involves changing the existing Category I codes for the primary service, for example adding slide digitization description to the descriptor of an existing service, the revised Category I code very well may end up at the same payment amount (without any additional associated amount for digitization). Additionally, alterations of one code may prompt re-evaluation of other codes within the code family. This is not generally a risk with Category III codes.
Finally, the RUC and PFS processes driving Category 1 rate determination do not have the flexibility of local payment rate determinations of Category III codes by Medicare Administrative Contractors.
Opportunities for Payment Outside the Traditional AMA/RUC-based Category 1 code Pathway
CMS has demonstrated its authority in several ways for various technologies, including AI software based technologies, that provide potential pathways for digital pathology reimbursement and exemplify how CMS diverges from AMA recommendations and coding at times, particularly for novel technologies and those involving software services.
- CMS has established national payment rates for Category III codes. As previously mentioned, the AMA does not engage in a RUC process for Category III codes, but CMS has shown a prior willingness to establish national payment rates for Category III codes in recent years. CMS has established national payment rates for glucose monitoring (0446T, 0447T, and 0448T) as well as for non-invasive Fractional Flow Reserve (e.g. HeartFlow) (0503T), and electroretinography (ERG) with interpretation and report (0509T).
- CMS may deviate from RUC guidance for valuation determinations for physician services. For CY2024, the four Category III codes comprising the HeartFlow service,as mentioned above (0501T-0503T), are being replaced by a single Category 1 code (7X005) when the service is provided in a physician office-based setting, but CMS did not follow the RUC recommendation for pricing the technical component of the Category 1 code and instead based the technical component amount on the legacy price CMS gave the Category III code (0503T) in 2018 under the Hospital Outpatient Prospective Payment System (a national price reflecting the cost of the software analysis which was based on a crosswalk to an existing test (93457)). Of note, the currently proposed payment rate for the professional component of this service is lower than MAC assigned payment rates in a number of jurisdictions.
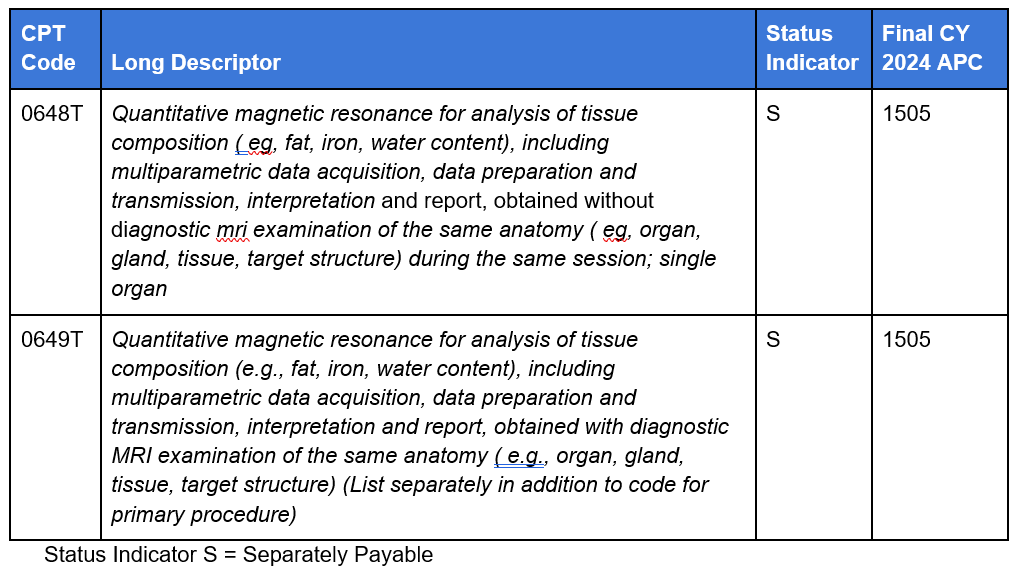
- For AI software technologies under the Hospital Outpatient Prospective Payment System (OPPS), CMS provides separate APC payment for the “add on” Category III code (not packaged) equal to the APC payment for the Category III code for the standalone imaging service code. In CY2022, CMS ruled that “Software as a Service” add-on codes are to be assigned to identical APCs and have the same status indicator assignments (separately paid) as the companion standalone codes.” Liver Multi-Scan, Optellum LCP and Quantitative Magnetic Resonance Cholangiopancreatography (QMCPR) had add-on codes that were therefore paid separately and equally to the standalone service. However, the current code construction of the slide digitization codes is such that there is no stand alone counterpart, but only add-on codes to distinct primary service codes. For other AI software services to take advantage of the opportunity for separate APC payment under OPPS, a similarly structured pair of codes, different from the current format of the slide digitization Category III add on codes, would need to be adopted by the AMA as described below and as shown in the LIver Multiscan example (Figure 6):
- A standalone code (e.g., 0648T) reflecting the description for the primary imaging service and the software add on service (for the payment of the primary service)
- A separately reported ancillary code (e.g., 0649T) reflecting the description of the primary imaging service and the software add on service (that also includes an instruction to list separately from the primary procedure).
4. CMS can establish alternative HCPCS Level II codes not aligned with codes established by the AMA which may affect reporting of slide digitization. Differences between the AMA and CMS coding policies have created barriers to correct coding for digital pathology services, specifically in the case of digitization coding for prostate biopsies. Prostate biopsy is a high frequency procedure in the U.S. In order to track utilization of prostate biopsies and curb biopsy overreporting made more possible by the the AMA code requirements and description, CMS chose not to use the AMA code and instead created a HCPCS Level II code, G0416 Surgical pathology, gross and microscopic examinations, for prostate needle biopsy, any method. The AMA does not generally develop add-on codes for HCPCS Level II codes it has not created. Therefore, the absence of an add-on code for slide digitization for G0416 has created significant barriers to correctly recording slide digitization of prostate cancer cases on claims to Medicare and private payers who have adopted the HCPCS Level IIcode.
The point of the above examples is to demonstrate that there are many potential pathways that CMS can follow if it chooses to assign payment or coding to digital pathology/AI services, and that obtaining payment for slide digitization and AI technologies is not dependent on converting Category III codes to Category 1 codes. These alternative pathways demonstrated by CMS highlight the importance of advocacy to influence coding and payment policy outcomes that yield appropriate valuation of digital pathology services.
How is the DPA Reimbursement Task Force Making a Difference?
The DPA is playing an active role in seeking policy actions that appropriately reimburse digital pathology and AI services and has recently initiated engagement with CMS on these issues. The DPA reimbursement Task Force is meeting with CMS this quarter to introduce the DPA organization, educate CMS on the value of digital pathology/AI, and advocate for key policy changes to:
- Remediate payment policies in which digital pathology AI services are currently packaged into the payment for a primary service; and
- Establish appropriate and separate valuation of digital pathology services distinct from the primary pathology service (e.g., by assigning payment rates to existing Category II codes).
The DPA Reimbursement Task Force is collaborating with other stakeholder organizations who are in alignment with DPA to spearhead or jointly sign on to policy advocacy letters. The Task Force plans to submit comment letters to CMS and Medicare Administrative Contractors in response to regulatory, coverage, coding and payment policies that impact digital pathology/AI such as those put forth in the proposed and final rules for the Physician Fee Schedule and the Hospital Outpatient Prospective Payment System. The DPA also plans to initiate or support coding initiatives that maximize the opportunities for reimbursement that align with CMS evolving policies.
How Can Digital Pathology Stakeholders Help?
The DPA encourages its members and the pathology community to monitor and understand the implications of AMA coding developments and CMS policy and rulings that affect coverage, coding and payment policy for digital pathology and AI software services.
CMS needs data if they are going to assign payment rates. In the case of Category III codes in which CMS will not use or obtain data from the RUC to determine the practice expense and/or the technical component, then it is up to other stakeholders in the industry to (1) provide that data, similar to the information the RUC provides (e.g., in the form of invoices for equipment and time allocation/time requirements) or (2) suggest crosswalk codes that approximate the additional capital expense and time associated with slide digitization. Such information can be provided proactively or in response to CMS requests for industry input and comment.
In a world of rising health care costs, it is critical for the digital pathology community to show that any innovation associated with new costs brings value. Stakeholders must communicate that value so that policy makers and payers are aware of the value digital pathology brings.
******
The DPA Reimbursement Task Force pledges to contribute actively to the momentum towards obtaining reimbursement for digital pathology services. The DPA strives to build an effective and unified advocacy effort for increasing adoption of digital pathology/AI and ensuring reimbursement of these services. Digital pathology’s reimbursement future has yet to be charted, and the DPA will continue to seek ways to influence coding, coverage and payment decision-making by policy makers on behalf of its members.
Please contact the DPA Reimbursement Task force info@digitalpathologyassociation.org
******
Figure 1
Coding Systems
Applicable to Pathology Services, Laboratory Tests, and Digital Pathology/AI Technologies
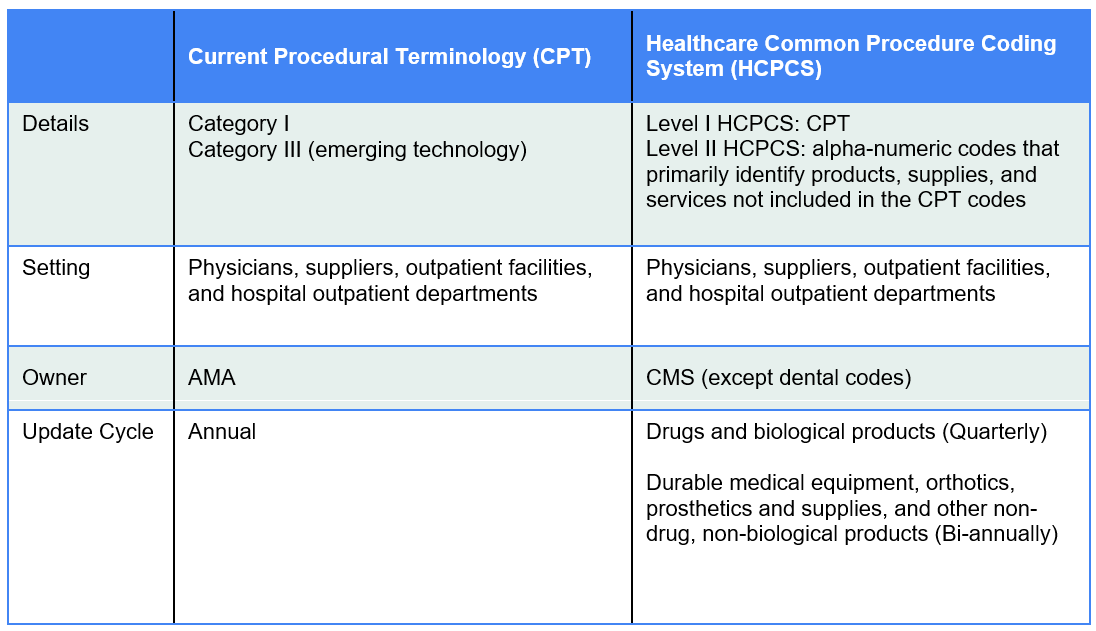
Figure 2
Formula for Physician Payment Rate Under the Medicare Physician Fee Schedule
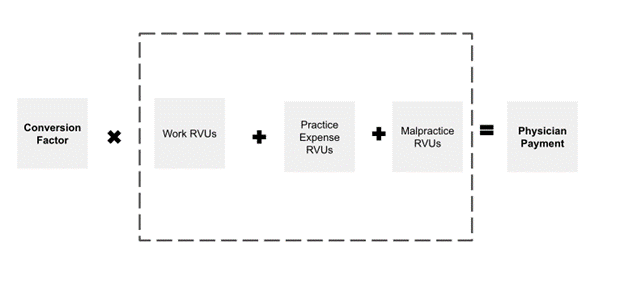
Figure 3
Inputs to Physician Payment Rate Determination
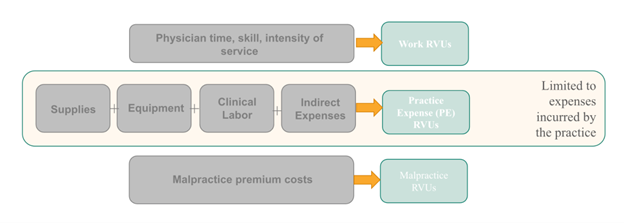
Figure 4
Medicare Prospective Payment Systems
Applicable to Pathology Services, Laboratory Tests, and Digital Pathology/AI Technologies
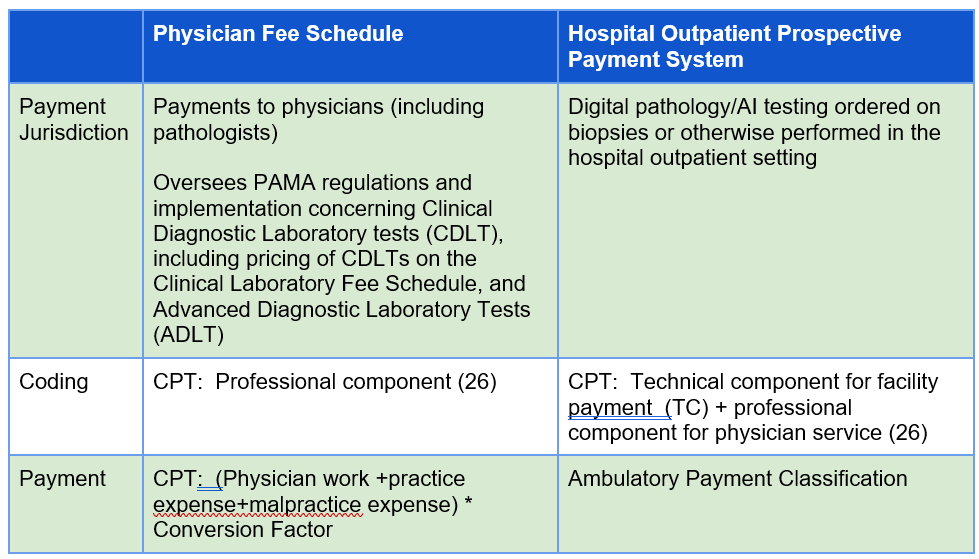
Figure 5
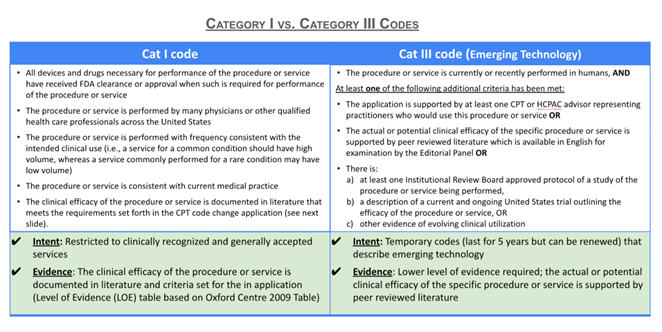
Figure 6
Liver Multiscan (Hospital Prospective Payment System 2024)
Disclaimer: In seeking to foster discourse on a wide array of ideas, the Digital Pathology Association believes that it is important to share a range of prominent industry viewpoints. This article does not necessarily express the viewpoints of the DPA; however, we view this as a valuable point with which to facilitate discussion.
Please log in to your DPA profile to submit comments